A weighted piston (total mass of 17.2 kg), resting on stops in a cylinder (radius of 0.05 m) divides a container into two regions. 0.075 kg of saturated liquid water at 50 C is contained beneath the piston. The rest of the cylinder and container is evacuated. Heat is applied to the water until the piston is lifted off of the stops and moved a distance () of 0.6 m. As the piston reaches the mouth of the cylinder, the hermetic seal is broken and the cylinder contents can now expand to fill the remainder of the evacuated space. At this instant, no additional heat is added to or removed from the system. When the system fully equilibrates, its absolute pressure is measured to be 0.01 MPa.
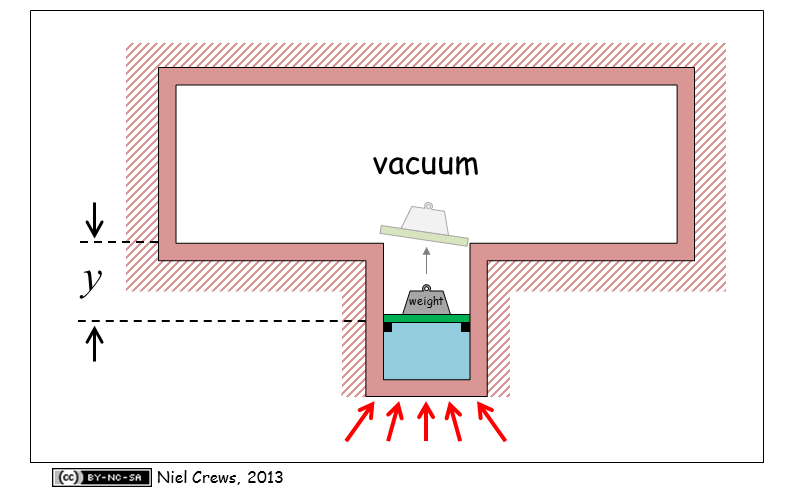
a) What is the state of the water when the piston begins to rise off of the stops?
b) What is the water temperature when the piston reaches the mouth of the cylinder? C
You can earn partial credit on this problem.